Instruction Manual Clarity II T56
PN-51-T56 May 2012
32 Appendix
Appendix
This procedure describes how to verify linearity between turbidity and TSS.
1. Collect a sample of the process liquid-you may need 10 L or more if you use the Clarity II for measuring turbidity. If
you use a laboratory turbidimeter, you will need less volume. The Clarity II requires about 500 mL for the measure-
ment; laboratory turbidimeters require 50 mL or less. Verify that the turbidity of the sample is less than 200 NTU.
Store in a clean bottle.
2. Filter a portion of the sample to obtain at least 5 L of dilution liquid. The filtrate is needed to dilute the sample in
subsequent steps. Verify that the turbidity of the dilution water is low. If filtering the sample is impractical, use
deionized water for dilution.
3. Measure the total suspended solids (TSS) in the sample obtained in step 1. Thoroughly mix the sample before
withdrawing liquid. A magnetic stirrer is best, but inverting the sample repeatedly for about a minute works, too.
Avoid violent shaking or mixing. Refer to any standard reference work on water and wastewater testing for the
procedure for determining TSS.
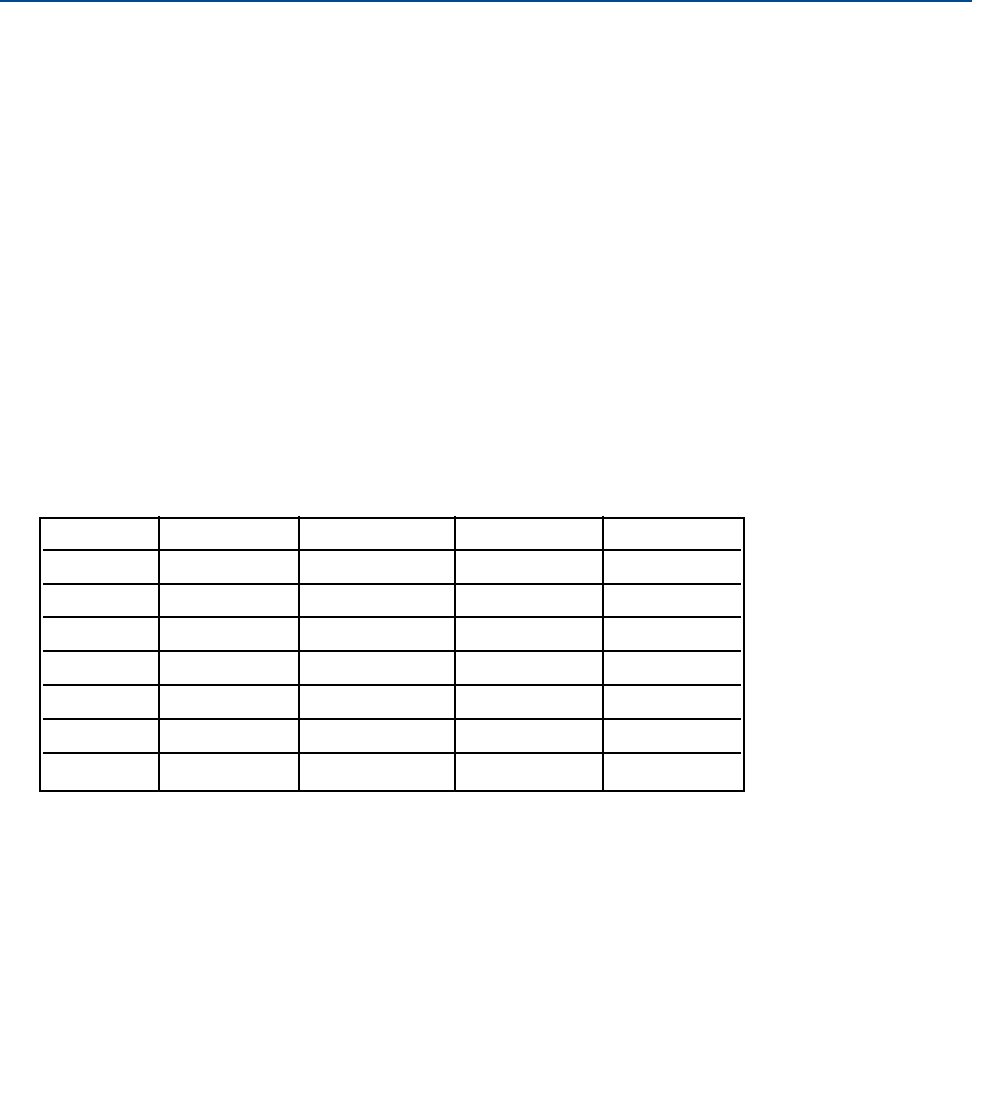
4. Dilute the sample from step 1, by a factor of 0.9, 0.7, 0.5, 0.3, and 0.1. See the table for recommended volumes.
Measure TSS and turbidity for each dilution. For lower TSS values, use a larger volume of sample.
5. Plot the data obtained in step 4, with turbidity on the y-axis and TSS on the x-axis. Fit the best straight line to the
data.
6. Locate two points (P1 and P2) on the line separated as much as possible. Read the ppm and NTU value for each
point and enter these into the analyzer. See Section 6.5.2.
Dilution Volume of Final Volume for Volume for
factor stock, mL volume, mL Clarity II, mL TSS, mL
1.00 -- -- 500 50 - 250
0.9 900 1000 500 50 - 250
0.7 700 1000 500 50 - 250
0.5 500 1000 500 50 - 250
0.3 300 1000 500 50 - 250
0.1 100 1000 500 50 - 250